Quality
Quality at Medicamen
We maintain stringent control systems and procedures to ensure compliance with CGMP standardsWe develop innovative and high-quality products drawing strength from our R&D team, which is not only futuristic in its ideas but also thoughtful towards humanity.
QUALITY ASSURANCE
A comprehensive quality assurance programme at every stage of procurement, manufacture, packing & distribution ensure that products not only meet but also surpass pharmacopoeial requirements. Stringent control systems & procedures ensure compliance with cGMP standards. Our Quality assurance dept. comprise of a separate department IPQA which monitors in process controls to assure inbuilt quality in the product.

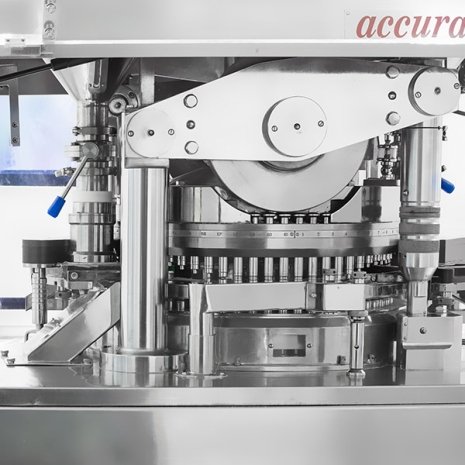
QUALITY CONTROL
We ensure the quality of starting and packaging materials by following Specifications & Standard Test Procedures. Our Quality control dept. is responsible for all sampling & analysis work related to raw material, in -process and finished goods validation samples & stability samples and aim at carrying out all requirements of current Good Laboratory Practices (cGLPs). We ensure that the incoming, in-process and final inspection is done as per documented procedures.